Comprehensive Strategies for the Cleaning and Disinfection of Endoscopes
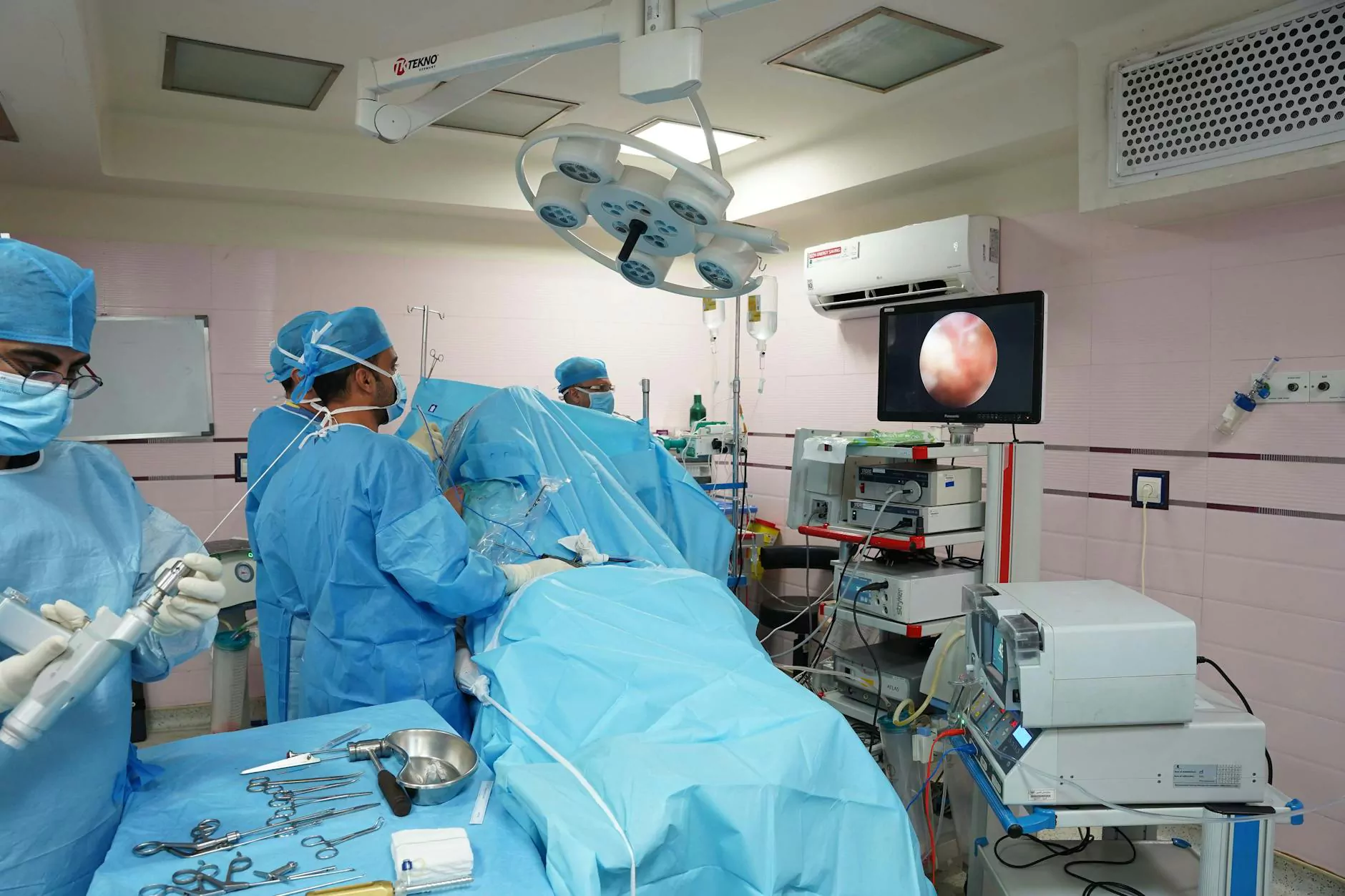
Introduction: The Critical Importance of Proper Cleaning and Disinfection of Endoscopes
In the rapidly advancing field of healthcare, the use of endoscopes has become vital for accurate diagnosis and minimally invasive treatments. However, the effectiveness of endoscopic procedures relies heavily on maintaining the highest standards of hygiene, particularly through meticulous cleaning and disinfection of endoscopes. Proper sterilization protocols not only safeguard patient health by preventing cross-contamination and infection transmission but also uphold the integrity and longevity of the endoscopic instruments.
This comprehensive guide delves into the essential aspects of endoscope cleaning and disinfection, emphasizing methodologies, best practices, innovative technologies, and industry standards. Whether you are a healthcare professional, a medical supplies provider, or a facility manager, understanding these critical components ensures safety, compliance, and optimal operational efficacy.
The Necessity of Rigorous Cleaning and Disinfection of Endoscopes
Endoscopes are complex devices that come into direct contact with internal body tissues and fluids. Their intricate design, comprising channels, valves, and movable parts, creates numerous niches where biological debris and pathogens can hide. Without rigorous cleaning, these residual contaminants pose significant risks, including:
- Nosocomial infections: contamination can lead to infections such as pneumonia, bacteremia, and other healthcare-associated infections (HAIs).
- Cross-infection risks: improper disinfection may facilitate pathogen transfer between patients.
- Compromised diagnostic accuracy: biofilm buildup and debris can impair imaging and instrument functionality.
Therefore, consistent adherence to validated cleaning protocols is fundamental for patient safety and maintaining clinical excellence.
Understanding the Endoscope Cleaning & Disinfection Process
Step 1: Pre-Cleaning Immediately After Use
The first step involves pre-cleaning immediately after the procedure, which helps prevent drying and hardening of biological material. This typically includes:
- Wiping the exterior with a lint-free cloth soaked in enzymatic detergent.
- Flushing all channels with detergent solution to remove debris.
- Gentle brushing of accessible channels and components as needed.
Step 2: Mechanical Cleaning
Mechanical cleaning utilizes specialized endoscope reprocessing machines or manual cleaning methods, involving:
- High-pressure flushing of channels with enzymatic detergents.
- Use of ultrasonic cleaners to dislodge residual biofilms.
- Use of compatible cleaning brushes for internal parts.
Step 3: Rinsing
Thorough rinsing with sterile or filtered water ensures removal of detergent residues that could impact disinfection efficacy.
Step 4: Disinfection and Sterilization
The core of the process, disinfection of endoscopes, involves various methods depending on the type of equipment and level of sterilization required:
- High-level disinfection (HLD): Utilizes chemical disinfectants like glutaraldehyde, orthophthalaldehyde (OPA), or peracetic acid to eliminate all microorganisms except bacterial spores.
- Sterilization: Employs methods like ethylene oxide (EO) gas, low-temperature hydrogen peroxide gas plasma, or peracetic acid sterilizers for complete sterilization of critical components.
Step 5: Drying and Storage
Proper drying using filtered, dried air prevents microbial growth and biofilm formation. Storage in a clean, ventilated cabinet preserves the endoscope’s condition and readiness for future use.
Best Practices for Effective Cleaning and Disinfection of Endoscopes
- Adherence to Manufacturer Instructions: Always follow the specific reprocessing guidelines provided by the endoscope manufacturer.
- Use of Validated Processes: Ensure that cleaning and disinfection procedures are validated and regularly monitored through quality assurance programs.
- Training and Competency: Regular training for staff involved in endoscope reprocessing is crucial to maintain high standards.
- Documentation and Traceability: Maintain detailed logs of cleaning cycles, disinfectants used, and maintenance records for accountability and regulatory compliance.
- Infrastructure Readiness: Install and maintain appropriate water filtration, ventilation, and cleaning equipment compliant with health standards.
- Routine Maintenance: Regularly inspect, test, and service reprocessing devices to ensure optimal functioning.
Implementing these best practices promotes not only patient safety but also operational efficiency and regulatory conformity.
Emerging Technologies Enhancing Endoscope Reprocessing
The future of cleaning and disinfection of endoscopes is driven by innovative technologies that improve efficacy, safety, and ease of use:
- Automated Endoscope Reprocessors (AERs): These machines standardize cleaning cycles, reduce human error, and ensure consistent results.
- Single-Use Endoscopes and Accessories: While currently limited, disposable options eliminate cross-contamination risks associated with traditional reusable devices.
- Advanced Chemical Disinfectants: New formulations with rapid action, fewer residues, and environmental benefits are continuously developed.
- Biofilm Disruption Technologies: Devices that target microbial biofilms enhance sterilization outcomes, especially in hard-to-reach channels.
In integrating cutting-edge tools, healthcare facilities can achieve higher standards of hygiene, operational safety, and cost-effectiveness.
Regulatory Standards and Compliance for Endoscope Reprocessing
Ensuring that cleaning and disinfection of endoscopes meet industry regulations is critical. Key standards include:
- CDC Guidelines: The Centers for Disease Control and Prevention provide comprehensive protocols for healthcare-associated infection prevention.
- ISO Standards: International Organization for Standardization guidelines specify requirements for medical device reprocessing.
- HTM (Healthcare Technical Memoranda): UK standards detailing best practices in medical equipment sterilization and hygiene.
- FDA Regulations: Ensuring compliance for sterilization devices and processes used in the United States.
Adherence to these standards ensures legal compliance, enhances patient safety, and fosters trust in healthcare services.
The Role of Medalkan in Supporting High-Quality Endoscope Disinfection
At medalkan.com, we specialize in providing advanced medical supplies and disinfection solutions tailored for healthcare environments. Our product range includes:
- High-grade enzymatic detergents for pre-cleaning
- Validated chemical disinfectants for high-level disinfection and sterilization
- State-of-the-art automated reprocessing equipment
- Comprehensive sterilization and hygiene accessories
Partnering with Medalkan ensures that your facility employs the most effective, compliant, and innovative solutions for the cleaning and disinfection of endoscopes. Our expert team is committed to supporting your infection control programs with solutions that meet or exceed industry standards.
Conclusion: Elevating Endoscope Hygiene Standards for Safer Healthcare
The intricate process of cleaning and disinfection of endoscopes is a cornerstone of patient safety and effective clinical practice. With the advent of advanced technologies, stringent standards, and dedicated professional oversight, healthcare providers are better equipped than ever to prevent infections and ensure the longevity of their endoscopic equipment.
Continuous education, adherence to validated protocols, and leveraging innovative solutions from trusted suppliers like Medalkan are vital steps toward excellence in medical hygiene standards. Prioritizing rigorous reprocessing practices not only safeguards your patients but also enhances the reputation and operational efficiency of your healthcare facility.
Investing in quality reprocessing processes and equipment is an investment in quality patient care and compliance, establishing your facility as a leader in healthcare safety and innovation.
© 2024 Medalkan. All rights reserved. | Visit our website









